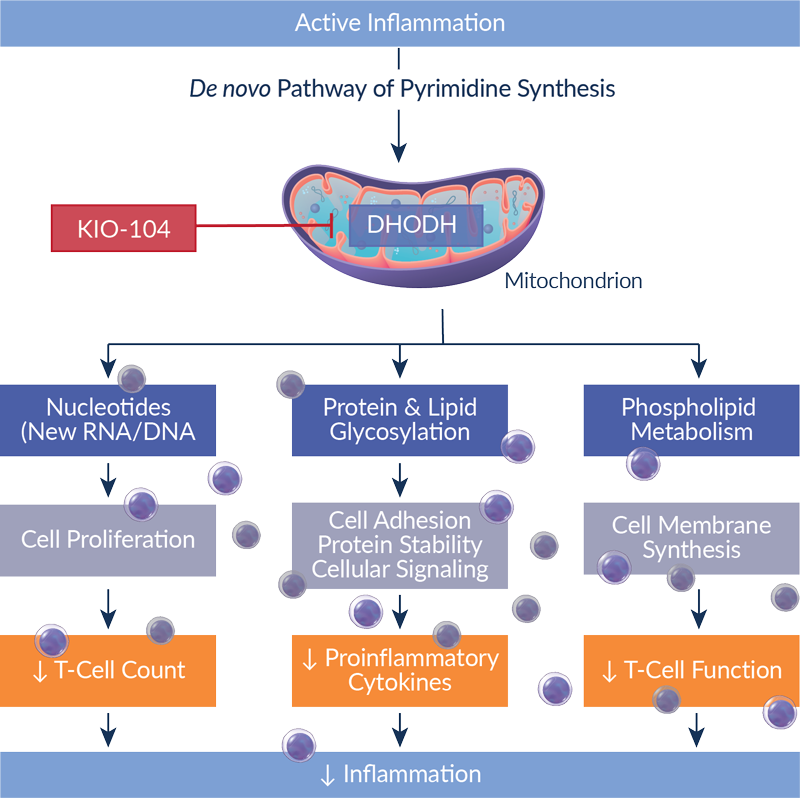
KIO-104 works by suppressing certain T-cells and cytokines in the eye that drive damaging inflammation. Specifically, KIO-104 inhibits the mitochondrial enzyme DHODH, which plays a crucial role in the synthesis of key building blocks of DNA and RNA. Without these, T-cell replication is significantly reduced. These building blocks also serve as key cofactors required for T-cell function, thus having fewer building blocks dampens the T-cell’s ability to drive inflammation.
The Phase 2 KLARITY study will explore KIO-104 in patients with conditions including posterior non-infectious uveitis, diabetic macular edema, and other retinal inflammatory diseases. For more information, visit clinicaltrials.gov